Nature has some very elegant and efficient solutions to the problems of making nanoscale structures, exploiting the self-assembling properties of information-containing molecules like proteins to great effect. A very promising approach to nanotechnology is to use what biology gives us to make useful nanoscale products and devices. I spent Monday visiting a nanotechnology company that is doing just that. Nanomagnetics is a Bristol based company (I should disclose an interest here, in that I’ve just been appointed to their Science Advisory Board) which exploits the remarkable self-assembled structure of the iron-storage protein ferritin to make nanoscale magnetic particles with uses in data storage, water purification and medicine.
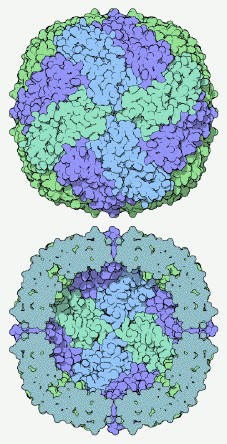
The illustration shows the ferritin structure; 24 individual identical protein molecules come together to form a hollow spherical shell 12 nm in diameter. The purpose of the molecule is to store iron until it is needed; iron ions enter through the pores and are kept inside the shell – given the tendency of iron to form a highly insoluble oxide, if we didn’t have this mechanism for storing the stuff our insides would literally rust up. Nanomagnetics is able to use the hollow shell that ferritin provides as a nanoscale chemical reactor, producing nanoparticles of magnetic iron oxide or other metals of great uniformity in size, and with a protein coat that both stops them sticking together and makes them biocompatible.
One simple, but rather neat, application of these particles is in water purification, in a process called forward osmosis. If you filled a bag made of a nanoporous membrane with sugar syrup, and immersed the bag in dirty water, water would be pulled through the membrane by the osmotic pressure exerted by the concentrated sugar solution. Microbes and contaminating molecules wouldn’t be able to get through the membrane, if its pores are small enough, and you end up with clean sugar solution. There’s a small company from Oregon, USA, HTI , which has commercialised just such a product. Essentially, it produces something like a sports drink from dirty or brackish water, and as such it’s started to prove its value for the military and in disaster relief situations. But what happens if you want to produce not sugar solution, but clean water? If you replace the sugar by magnetic nanoparticles then you can sweep the particles away with a magnetic field and then use them again to produce another batch of water, producing clean water from simple equipment with only a small cost in energy.
The illustration of ferritin is taken from the Protein Database’s Molecule of the Month feature. The drawing is by David S. Goodsell, based on the structure determined by Lawson et al., Nature 349 pp. 541 (1991).